The IBC oversees compliance with university, local, state, and federal regulations governing biological safety and security, in compliance with the NIH Guidelines for Research Involving Recombinant DNA Molecules.
Principal Investigators (PIs) planning to carry out research or teaching which involve any of the following biological hazards must submit an IBC Registration Form to the IBC for review:
- Recombinant DNA
- Genetically Modified Organisms
- Gene Drive Modified Organisms
- Transgenic Animals and/or Plants
- Micro-organisms known to cause any level of disease in healthy humans
- Materials derived from human and non-human primates
- Blood
- Body fluids
- Tissues
- Cells
- Acute Biological Toxins with an LD50 of less than 100 micrograms per kilogram of body weight in vertebrates
- Select Agents or Toxins
- Proposed research subject to the United States Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern
- Nanomaterials (Effective 1/1/2025)
- Highly toxic: (Effective 1/1/2025)
- Chemicals
- Chemical carcinogens/mutagens, and/or
- Cytotoxic drugs
Please see our Definitions website for more information.
Training
Required CITI Training
- Initial Biosafety
- Lab Chemical Safety
- Other Biosafety and Biosecurity modules relevant to your specific research focus
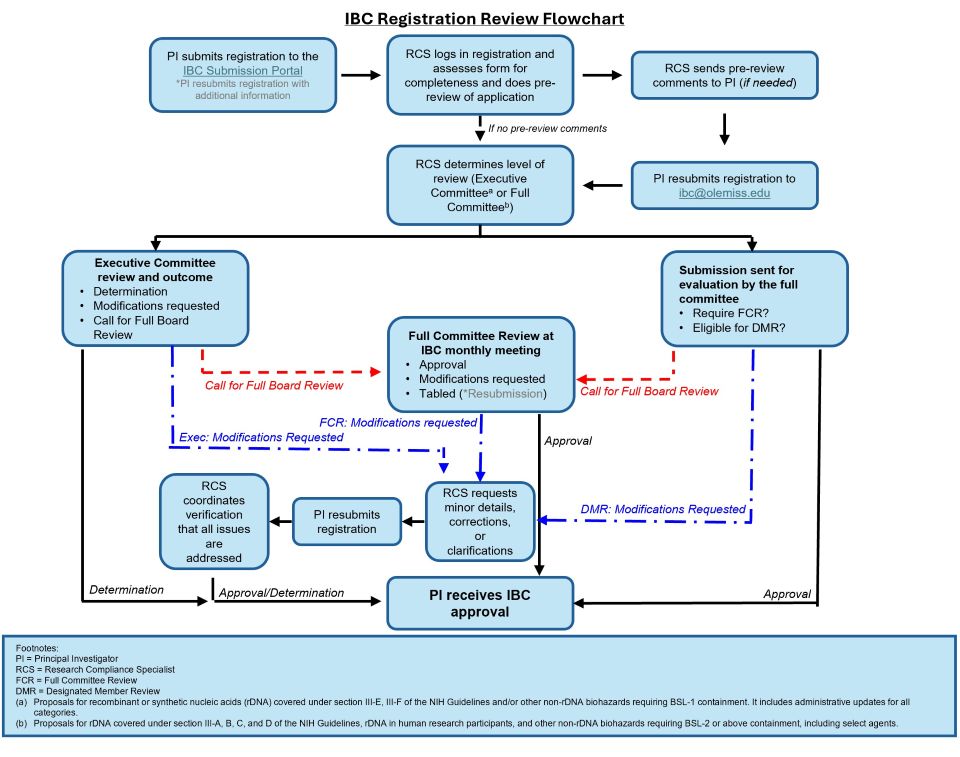
Procedures for Reviewing IBC Registrations
- Registrations involving either recombinant DNA research or the use of biological materials has been assessed to require a level of containment of Biosafety Level 1 (BSL1) or its equivalent (Group I, Class 1, or Low Risk), the Registration may be approved or determined Exempt by the IBC Executive Committee (IBC Chair and at least one other voting member).
- Any member of the IBC Executive Committee who reviews the Registration may request review by the full committee
- Registrations reviewed and approved under these provisions will be reported in the activity report to the IBC at its next convened meeting. Copies of Registrations will be available to all IBC members
- Registrations that require containment procedures at or above Biosafety Level 2 (BSL2) are reviewed by the full IBC at a scheduled meeting.
Procedures for Continuing Review of IBC Registrations
- The IBC Office will inform the Principal Investigator that the current IBC Registration is about to expire and that a renewal or modification is required. Each registration is approved for a maximum of three years.
- Prior to expiration, the PI must submit a new Registration form for review. We recommend that you submit your renewal at least 60 days before the expiration date to ensure that there is no lapse in coverage.
Procedures for Amending an IBC Registration
To submit an amendment (to procedures or personnel), please complete the amendment form and incorporate the changes (highlighted) in the approved registration.
- Minor modifications to the approved registration - such as disinfectant changes, use of same agent in a different form, or changes in vector or host organisms that do not alter classification of the experiment or safety of the registration require that an Amendment be submitted to the IBC for review.
- The amendment will be submitted to the committee to determine if it will be reviewed by Designated Member Review (DMR) or reviewed by the Full Committee.
- Approved amendments will be reported to the IBC at the next regular scheduled meeting.
- Major modifications to the approved registration - such as changes in PI, changes/additions to chemical or biological agents, changes in animal model, or use of vector or host organisms that change classification of the experiment require completion of an Amendment and review by the IBC at a scheduled meeting. In some cases, the IBC may require a new Registration Form.